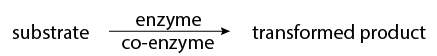Chemical Reactions
Chemical reactions are continually taking place in the body. They are a normal aspect of life, participating in the:
Enzymes
Enzymes are the catalysts for nearly all biochemical reactions in the body. Without these enzymes, essential biotransformation reactions would take place slowly or not at all, causing major health problems.
Did you know?
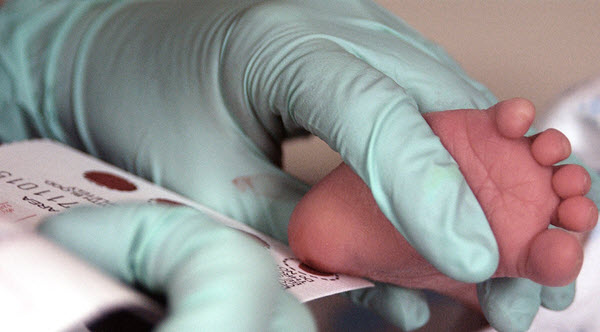
Phenylketonuria (PKU) is the genetic condition in which the enzyme that biotransforms phenylalanine to tyrosine (another amino acid) is defective. As the result, phenylalanine can build up in the body and cause severe mental retardation. Babies are routinely checked at birth for PKU. If they have PKU, they need to follow a special diet to restrict the intake of phenylalanine in infancy and childhood.
- Building up of new tissue.
- Tearing down of old tissue.
- Conversion of food to energy.
- Disposal of waste materials.
- Elimination of toxic xenobiotics.
Enzymes
Enzymes are the catalysts for nearly all biochemical reactions in the body. Without these enzymes, essential biotransformation reactions would take place slowly or not at all, causing major health problems.
Did you know?
Phenylketonuria (PKU) is the genetic condition in which the enzyme that biotransforms phenylalanine to tyrosine (another amino acid) is defective. As the result, phenylalanine can build up in the body and cause severe mental retardation. Babies are routinely checked at birth for PKU. If they have PKU, they need to follow a special diet to restrict the intake of phenylalanine in infancy and childhood.
Figure 1. Phenylketonuia (PKU) testing in an infant
(Image Source: Wikimedia Commons, obtained under Public Domain license. Author: U.S. Air Force Photographic Archives)
(Image Source: Wikimedia Commons, obtained under Public Domain license. Author: U.S. Air Force Photographic Archives)
These enzymatic reactions are not always simple biochemical reactions. Some enzymes require the presence of cofactors or coenzymes in addition to the substrate (the substance to be catalyzed) before their catalytic activity can be exerted. These co-factors exist as a normal component in most cells and are frequently involved in common reactions to convert nutrients into energy (vitamins are an example of co-factors). It is the drug or chemical transforming enzymes that hold the key to xenobiotic transformation. The relationship of substrate, enzyme, coenzyme, and transformed product can be shown as:
Most biotransforming enzymes are high molecular weight proteins, composed of chains of amino acids linked together by peptide bonds. A wide variety of biotransforming enzymes exist. Most enzymes will catalyze the reaction of only a few substrates, meaning that they have high specificity. Specificity is a function of the enzyme's structure and its catalytic sites. While an enzyme may encounter many different chemicals, only those chemicals (substrates) that fit within the enzyme's convoluted structure and spatial arrangement will be locked on and affected. This is sometimes referred to as the "lock and key" relationship.
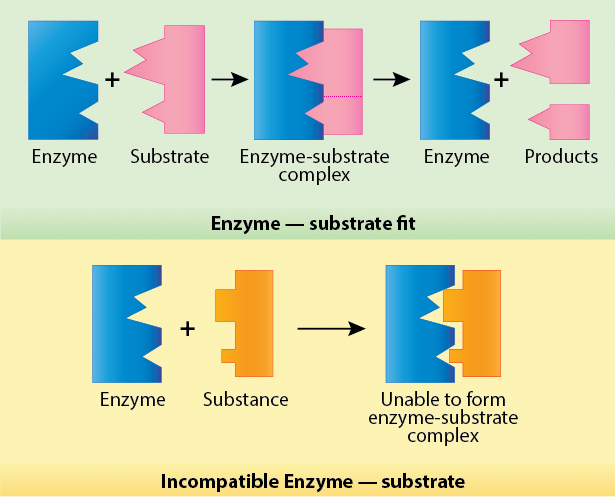
As shown in Figure 2, when a substrate fits into the enzyme's structure, an enzyme-substrate complex can be formed. This allows the enzyme to react with the substrate with the result that two different products are formed. If the substrate does not fit into the enzyme ("incompatible"), no complex will be formed and thus no reaction can occur.
As shown in Figure 2, when a substrate fits into the enzyme's structure, an enzyme-substrate complex can be formed. This allows the enzyme to react with the substrate with the result that two different products are formed. If the substrate does not fit into the enzyme ("incompatible"), no complex will be formed and thus no reaction can occur.
Figure 2. If the substrate does not fit into the enzyme, no complex will be formed and no reaction will occur.
(Image Source: NLM)
(Image Source: NLM)
Enzyme Specificity
Enzymes range from having absolute specificity to broad and overlapping specificity. In general, there are three main types of specificity:
Enzyme Naming Convention
The names assigned to enzymes may seem confusing at first. However, except for some of the originally studied enzymes (such as pepsin and trypsin), a convention has been adopted to name enzymes. Enzyme names end in "ase" and usually combine the substrate acted on and the type of reaction catalyzed.
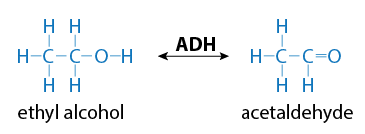
For example, alcohol dehydrogenase is an enzyme that biotransforms alcohols by the removal of a hydrogen. The result is a completely different chemical, an aldehyde or ketone.
The biotransformation of ethyl alcohol to acetaldehyde is depicted in Figure 3.
Enzymes range from having absolute specificity to broad and overlapping specificity. In general, there are three main types of specificity:
- Absolute — the enzyme will catalyze only one reaction. Examples:
- Formaldehyde dehydrogenase catalyzes only the reaction for formaldehyde.
- Acetylcholinesterase biotransforms the neurotransmitting chemical, acetylcholine.
- Group — the enzyme will act only on molecules that have specific functional groups, such as amino, phosphate, or methyl groups.
- For example, alcohol dehydrogenase can biotransform several different alcohols, including methanol and ethanol.
- Linkage — the enzyme will act on a particular type of chemical bond regardless of the rest of the molecular structure.
- For example, N-oxidation can catalyze a reaction of a nitrogen bond, replacing the nitrogen with oxygen.
- For example, N-oxidation can catalyze a reaction of a nitrogen bond, replacing the nitrogen with oxygen.
Enzyme Naming Convention
The names assigned to enzymes may seem confusing at first. However, except for some of the originally studied enzymes (such as pepsin and trypsin), a convention has been adopted to name enzymes. Enzyme names end in "ase" and usually combine the substrate acted on and the type of reaction catalyzed.
For example, alcohol dehydrogenase is an enzyme that biotransforms alcohols by the removal of a hydrogen. The result is a completely different chemical, an aldehyde or ketone.
The biotransformation of ethyl alcohol to acetaldehyde is depicted in Figure 3.
ADH = alcohol dehydrogenase, a specific catalyzing enzyme
Figure 3. Biotransformation of ethyl alcohol
(Image Source: NLM)
(Image Source: NLM)
|
Beneficial or Harmful?
At this point in ToxTutor you likely see that the transformation of a specific xenobiotic can be either beneficial or harmful, and perhaps both depending on the dose and circumstances. A good example is the biotransformation of acetaminophen (Tylenol®). When the prescribed doses are taken, the desired therapeutic response is observed with little or no toxicity. However, when excessive doses of acetaminophen are taken, hepatotoxicity can occur. This is because acetaminophen normally undergoes rapid biotransformation with the metabolites quickly eliminated in the urine and feces. At high doses, the normal level of enzymes may be depleted and the acetaminophen is available to undergo the reaction by an additional biosynthetic pathway, which produces a reactive metabolite that is toxic to the liver. For this reason, a user of Tylenol® is warned not to take the prescribed dose more frequently than every 4–6 hours and not to consume more than four doses within a 24-hour period. Biotransforming enzymes, like most other biochemicals, are available in a normal amount and in some situations can be "used up" at a rate that exceeds the body's ability to replenish them. This illustrates the frequently used phrase, the "dose makes the poison." |
Figure 4. Generic acetaminophen tablets
(Image Source: iStock Photos, ©) |
Biotransformation Reaction Phases
Biotransformation reactions are categorized not only by the nature of their reactions, for example, oxidation, but also by the normal sequence with which they tend to react with a xenobiotic. They are usually classified as Phase I and Phase II reactions.
Phase I reactions are generally reactions which modify the chemical by adding a functional structure. This allows the substance to "fit" into a second, or Phase II enzyme, so that it can become conjugated (joined together) with another substance.
Phase II reactions consist of those enzymatic reactions that conjugate the modified xenobiotic with another substance. The conjugated products are larger molecules than the substrate and generally polar in nature (water soluble). Thus, they can be readily excreted from the body. Conjugated compounds also have poor ability to cross cell membranes.
In some cases, the xenobiotic already has a functional group that can be conjugated and the xenobiotic can be biotransformed by a Phase II reaction without going through a Phase I reaction.
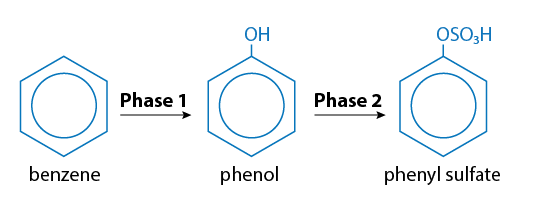
For example, phenol can be directly conjugated into a metabolite that can then be excreted. The biotransformation of benzene requires both Phase I and Phase II reactions. As illustrated in Figure 5, benzene is biotransformed initially to phenol by a Phase I reaction (oxidation). Phenol has a structure including a functional hydroxyl group that is then conjugated by a Phase II reaction (sulfation) to phenyl sulfate.
Biotransformation reactions are categorized not only by the nature of their reactions, for example, oxidation, but also by the normal sequence with which they tend to react with a xenobiotic. They are usually classified as Phase I and Phase II reactions.
Phase I reactions are generally reactions which modify the chemical by adding a functional structure. This allows the substance to "fit" into a second, or Phase II enzyme, so that it can become conjugated (joined together) with another substance.
Phase II reactions consist of those enzymatic reactions that conjugate the modified xenobiotic with another substance. The conjugated products are larger molecules than the substrate and generally polar in nature (water soluble). Thus, they can be readily excreted from the body. Conjugated compounds also have poor ability to cross cell membranes.
In some cases, the xenobiotic already has a functional group that can be conjugated and the xenobiotic can be biotransformed by a Phase II reaction without going through a Phase I reaction.
For example, phenol can be directly conjugated into a metabolite that can then be excreted. The biotransformation of benzene requires both Phase I and Phase II reactions. As illustrated in Figure 5, benzene is biotransformed initially to phenol by a Phase I reaction (oxidation). Phenol has a structure including a functional hydroxyl group that is then conjugated by a Phase II reaction (sulfation) to phenyl sulfate.
Figure 5. Biotransformation of benzene into phenol in Phase 1 (oxidation), which is then conjugated by a Phase 2 reaction (sulfation) to phenyl sulfate
(Image Source: NLM)
(Image Source: NLM)
Table 1 lists the major transformation reactions for xenobiotics broken into Phase I and Phase II reactions. These reactions are discussed in more detailed below.
Phase I Reactions
Phase I biotransformation reactions are simple reactions compared to Phase II reactions. In Phase I reactions, a small polar group (containing both positive and negative charges) is either exposed on the toxicant or added to the toxicant. The three main Phase I reactions are 1) oxidation; 2) reduction; and 3) hydrolysis.
Oxidation
Oxidation is a chemical reaction in which a substrate loses electrons. There are a number of reactions that can achieve the removal of electrons from the substrate.
- The addition of oxygen, or oxygenation, was the first of these reactions discovered and thus the reaction was named oxidation. However, many of the oxidizing reactions do not involve oxygen.
- The simplest type of oxidation reaction is dehydrogenation, which is the removal of hydrogen from the molecule.
- Another example of oxidation is electron transfer that consists simply of the transfer of an electron from the substrate.
Figure 6. Three types of oxidation reactions
(Image Source: NLM)
(Image Source: NLM)
The specific oxidizing reactions and oxidizing enzymes are numerous and several textbooks are devoted to this subject. Most of the reactions are described by the name of the reaction or enzyme involved. Some of these oxidizing reactions include:
Reduction
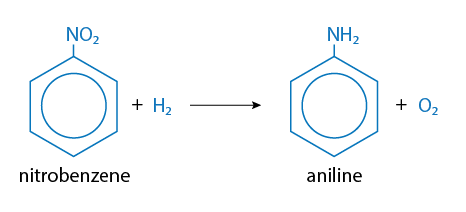
Reduction is a chemical reaction in which the substrate gains electrons. Reductions are most likely to occur with xenobiotics in which oxygen content is low. Reductions can occur across nitrogen-nitrogen double bonds (azo reduction) or on nitro groups (NO2). Frequently, the resulting amino compounds are oxidized which forms toxic metabolites. Some chemicals such as carbon tetrachloride can be reduced to free radicals, which are quite reactive with biological tissues. Thus, reduction reactions frequently result in activation of a xenobiotic rather than detoxification. An example of a reduction reaction in which the nitro group is reduced is illustrated in Figure 7.
- Alcohol dehydrogenation
- Aldehyde dehydrogenation
- Alkyl/acyclic hydroxylation
- Aromatic hydroxylation
- Deamination
- Desulfuration
- N-dealkylation
- N-hydroxylation
- N-oxidation
- O-dealkylation
- Sulphoxidation
Reduction
Reduction is a chemical reaction in which the substrate gains electrons. Reductions are most likely to occur with xenobiotics in which oxygen content is low. Reductions can occur across nitrogen-nitrogen double bonds (azo reduction) or on nitro groups (NO2). Frequently, the resulting amino compounds are oxidized which forms toxic metabolites. Some chemicals such as carbon tetrachloride can be reduced to free radicals, which are quite reactive with biological tissues. Thus, reduction reactions frequently result in activation of a xenobiotic rather than detoxification. An example of a reduction reaction in which the nitro group is reduced is illustrated in Figure 7.
Figure 7. Reduction reaction in which the nitro group is reduced (from NO2 to NH2) ** NO2 and NH2 written with subscripts as seen in the Figure above
There are fewer specific reduction reactions than oxidizing reactions. The nature of these reactions is also described by their name. Some reducing reactions include:
Hydrolysis
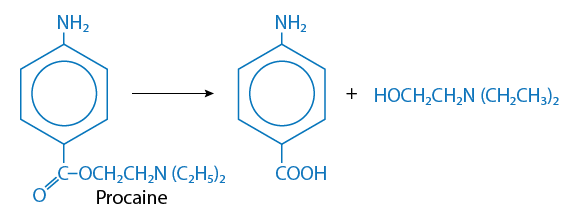
Hydrolysis is a chemical reaction in which the addition of water splits the toxicant into two fragments or smaller molecules. The hydroxyl group (OH-) is incorporated into one fragment and the hydrogen atom is incorporated into the other. Larger chemicals such as esters, amines, hydrazines, and carbamates are generally biotransformed by hydrolysis.
An example of hydrolysis is illustrated in the biotransformation of procaine (local anesthetic) which is hydrolyzed to two smaller chemicals (Figure 8).
- Azo reduction
- Dehalogenation
- Disulfide reduction
- Nitro reduction
- N-oxide reduction
- Sulfoxide reduction
Hydrolysis
Hydrolysis is a chemical reaction in which the addition of water splits the toxicant into two fragments or smaller molecules. The hydroxyl group (OH-) is incorporated into one fragment and the hydrogen atom is incorporated into the other. Larger chemicals such as esters, amines, hydrazines, and carbamates are generally biotransformed by hydrolysis.
An example of hydrolysis is illustrated in the biotransformation of procaine (local anesthetic) which is hydrolyzed to two smaller chemicals (Figure 8).
Figure 8. Hydrolysis of procaine
(Image Source: Adapted from Humboldt State University, Department of Chemistry. Author: Richard A. Paselk, Professor Emeritus.
(Image Source: Adapted from Humboldt State University, Department of Chemistry. Author: Richard A. Paselk, Professor Emeritus.
Toxicants that have undergone Phase I biotransformation are converted to metabolites that are sufficiently ionized, or hydrophilic, to be either eliminated from the body without further biotransformation or converted to an intermediate metabolite that is ready for Phase II biotransformation. The intermediates from Phase I transformations may be pharmacologically more effective and in many cases more toxic than the parent xenobiotic.
Phase II Reactions
A xenobiotic that has undergone a Phase I reaction is now a new intermediate metabolite that contains a reactive chemical group such as hydroxyl (-OH), amino (-NH2), and carboxyl (-COOH). Many of these intermediate metabolites do not possess sufficient hydrophilicity to permit elimination from the body. These metabolites must undergo additional biotransformation as a Phase II reaction.
Phase II reactions are conjugation reactions where a molecule normally present in the body is added to the reactive site of the Phase I metabolite. The result is a conjugated metabolite that is more water soluble than the original xenobiotic or Phase I metabolite. Usually, the Phase II metabolite is quite hydrophilic and can be readily eliminated from the body. The primary Phase II reactions are:
Glucuronide Conjugation
Glucuronide conjugation is one of the most important and common Phase II reactions. The glucuronic acid molecule is used in this reaction. It is derived from glucose, a common carbohydrate (sugar) that is the primary source of energy for cells. In this reaction, glucuronic acid is added directly to the toxicant or its phase I metabolite. The sites of glucuronidation reactions are substrates having an oxygen, nitrogen, or sulfur bond, which apply to a wide array of xenobiotics as well as endogenous substances, such as bilirubin, steroid hormones, and thyroid hormones.
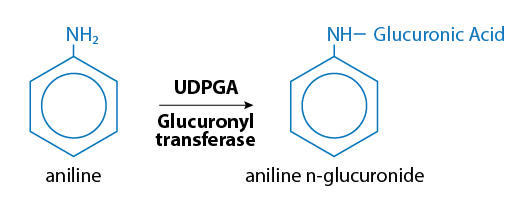
Glucuronidation is a pathway that conjugates xenobiotics at a high capacity ("high-capacity pathway"). Glucuronide conjugation usually decreases toxicity although there are some notable exceptions, for example, where it can result in producing carcinogenic substances. The glucuronide conjugates are generally quite hydrophilic and are excreted by the kidney or bile, depending on the size of the conjugate. The glucuronide conjugation of aniline is illustrated in Figure 9.
Phase II Reactions
A xenobiotic that has undergone a Phase I reaction is now a new intermediate metabolite that contains a reactive chemical group such as hydroxyl (-OH), amino (-NH2), and carboxyl (-COOH). Many of these intermediate metabolites do not possess sufficient hydrophilicity to permit elimination from the body. These metabolites must undergo additional biotransformation as a Phase II reaction.
Phase II reactions are conjugation reactions where a molecule normally present in the body is added to the reactive site of the Phase I metabolite. The result is a conjugated metabolite that is more water soluble than the original xenobiotic or Phase I metabolite. Usually, the Phase II metabolite is quite hydrophilic and can be readily eliminated from the body. The primary Phase II reactions are:
- Glucuronide conjugation – most important reaction (detailed below)
- Sulfate conjugation – important reaction (detailed below)
- Acetylation
- Amino acid conjugation
- Glutathione conjugation
- Methylation
Glucuronide Conjugation
Glucuronide conjugation is one of the most important and common Phase II reactions. The glucuronic acid molecule is used in this reaction. It is derived from glucose, a common carbohydrate (sugar) that is the primary source of energy for cells. In this reaction, glucuronic acid is added directly to the toxicant or its phase I metabolite. The sites of glucuronidation reactions are substrates having an oxygen, nitrogen, or sulfur bond, which apply to a wide array of xenobiotics as well as endogenous substances, such as bilirubin, steroid hormones, and thyroid hormones.
Glucuronidation is a pathway that conjugates xenobiotics at a high capacity ("high-capacity pathway"). Glucuronide conjugation usually decreases toxicity although there are some notable exceptions, for example, where it can result in producing carcinogenic substances. The glucuronide conjugates are generally quite hydrophilic and are excreted by the kidney or bile, depending on the size of the conjugate. The glucuronide conjugation of aniline is illustrated in Figure 9.
Figure 9. Glucuronide conjugation of aniline (which is used to make polyurethane, pharmaceuticals, and industrial chemicals)
(Image Source: NLM)
(Image Source: NLM)
Sulfate Conjugation
Sulfate conjugation is another important Phase II reaction that occurs with many xenobiotics. In general, sulfation decreases the toxicity of xenobiotics. Unlike glucuronic acid conjugates that are often eliminated in the bile, the highly polar sulfate conjugates are readily secreted in the urine. In general, sulfation is a low-capacity pathway for xenobiotic conjugation. Often glucuronidation or sulfation can conjugate the same xenobiotics.
Knowledge Check (Solutions on next page)
1) The substances in the body that accelerate chemical reactions are known as:
a) Amino acids
b) Enzymes
c) Substrates
2) The convention used to name specific enzymes consists of combining:
a) The substrate name with the type of chemical reaction
b) The target organ and the type of chemical reaction
c) The substrate name with the form of toxicity
3) Biotransformation reactions are classified as Phase I and Phase II. The basic difference is:
a) Phase I reactions conjugate a substrate whereas Phase II reactions oxidize the substance
b) Phase I reactions generally add a functional structure whereas Phase II reactions conjugate the substance
c) A Phase I reaction generally makes a substance more hydrophilic than a Phase II reaction
4) The difference between oxidation and reduction reactions is:
a) A substrate gains electrons from an oxidation reaction whereas it loses electrons by a reduction reaction
b) Oxygen is removed from a substrate in oxidation and added in the reduction reaction
c) A substrate losses electrons from an oxidation reaction whereas it gains electrons by a reduction reaction
5) Which conjugation reaction is the most common in the biotransformation of xenobiotics?
a) Amino acid conjugation
b) Glucuronide conjugation
c) Methylation