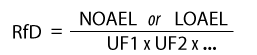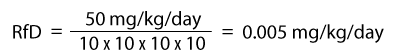Dose-Response Assessment
|
The dose-response assessment step of the risk assessment process quantitates the hazards that were identified in the previous step. It determines the relationship between dose and incidence of effects in humans. There are normally two major extrapolations required:
|
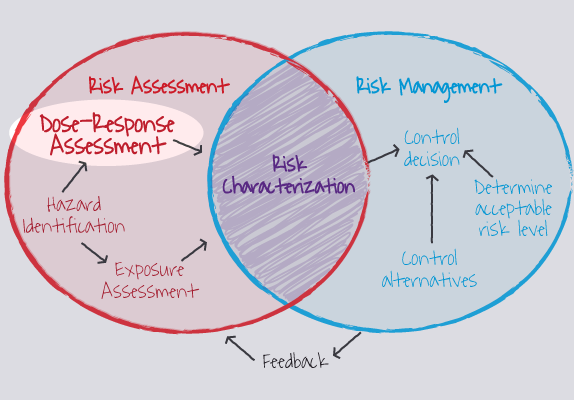
Figure 1. Dose-response assessment is a step in the risk assessment process
(Image Source: ORAU, ©) |
Carcinogen (Cancer) Risk Assessment
Cancer risk assessment involves two steps:
1. Qualitative Evaluation of Cancer Risk
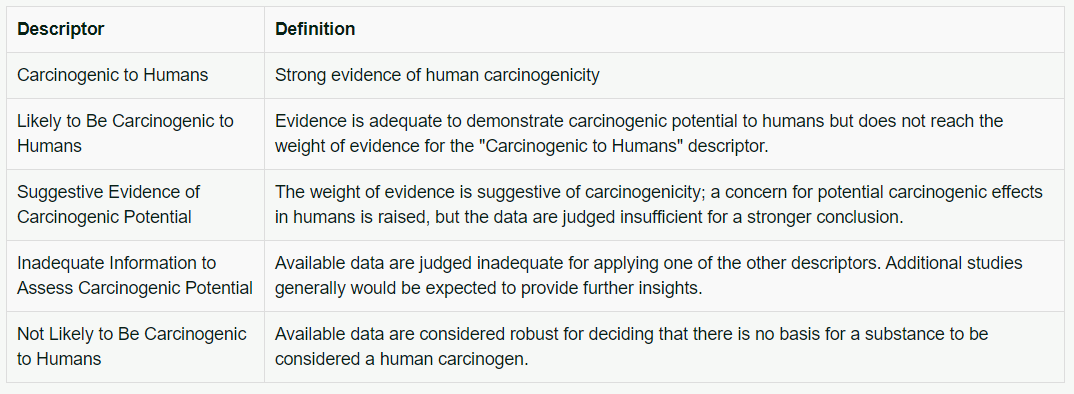
The EPA's cancer assessment procedures have been used by several Federal and State agencies. The Agency for Toxic Substances and Disease Registry (ATSDR) relies on EPA's carcinogen assessments. A substance is assigned to one of five descriptors shown below in Table 1.
Cancer risk assessment involves two steps:
- Perform qualitative evaluation of all epidemiology studies, animal bioassay data, and biological activity (for example, mutagenicity).The substance is classified as to its carcinogenic risk to humans based on the weight of evidence. If the evidence is sufficient, the substance may be classified as a definite, probable or possible human carcinogen.
- Quantitate the risk for those substances classified as definite or probable human carcinogens. Mathematical models are used to extrapolate from the high experimental doses to the lower environmental doses.
1. Qualitative Evaluation of Cancer Risk
The EPA's cancer assessment procedures have been used by several Federal and State agencies. The Agency for Toxic Substances and Disease Registry (ATSDR) relies on EPA's carcinogen assessments. A substance is assigned to one of five descriptors shown below in Table 1.
Cancer Data for Humans
The basis for sufficient human evidence is an epidemiology study that clearly demonstrates a causal relationship between exposure to the substance and cancer in humans.
The data are determined to be limited evidence in humans if there are alternative explanations for the observed effect.
The data are considered to be inadequate evidence in humans if no satisfactory epidemiology studies exist.
Cancer Data for Animals
An increase in cancer in more than one species or strain of laboratory animals or in more than one experiment is considered sufficient evidence in animals. Data from a single experiment can also be considered sufficient animal evidence if there is a high incidence or unusual type of tumor induced. Normally, however, a carcinogenic response in only one species, strain, or study is considered as only limited evidence in animals.
2. Quantitative Evaluation of Cancer Risk
When an agent is classified as a Human or Probable Human Carcinogen, it is then subjected to a quantitative risk assessment. For those designated as a Possible Human Carcinogen, the risk assessor can determine on a case-by-case basis whether a quantitative risk assessment is warranted.
The key risk assessment parameter derived from the EPA carcinogen risk assessment is the cancer slope factor. This is a toxicity value that quantitatively defines the relationship between dose and response. The cancer slope factor is a plausible upper-bound estimate of the probability that an individual will develop cancer if exposed to a chemical for a lifetime of 70 years. The cancer slope factor is expressed as mg/kg/day.
Linearized Multistage Model (LMS)
Mathematical models are used to extrapolate from animal bioassay or epidemiology data to predict low-dose risk. Most assume linearity with a zero threshold dose.
The basis for sufficient human evidence is an epidemiology study that clearly demonstrates a causal relationship between exposure to the substance and cancer in humans.
The data are determined to be limited evidence in humans if there are alternative explanations for the observed effect.
The data are considered to be inadequate evidence in humans if no satisfactory epidemiology studies exist.
Cancer Data for Animals
An increase in cancer in more than one species or strain of laboratory animals or in more than one experiment is considered sufficient evidence in animals. Data from a single experiment can also be considered sufficient animal evidence if there is a high incidence or unusual type of tumor induced. Normally, however, a carcinogenic response in only one species, strain, or study is considered as only limited evidence in animals.
2. Quantitative Evaluation of Cancer Risk
When an agent is classified as a Human or Probable Human Carcinogen, it is then subjected to a quantitative risk assessment. For those designated as a Possible Human Carcinogen, the risk assessor can determine on a case-by-case basis whether a quantitative risk assessment is warranted.
The key risk assessment parameter derived from the EPA carcinogen risk assessment is the cancer slope factor. This is a toxicity value that quantitatively defines the relationship between dose and response. The cancer slope factor is a plausible upper-bound estimate of the probability that an individual will develop cancer if exposed to a chemical for a lifetime of 70 years. The cancer slope factor is expressed as mg/kg/day.
Linearized Multistage Model (LMS)
Mathematical models are used to extrapolate from animal bioassay or epidemiology data to predict low-dose risk. Most assume linearity with a zero threshold dose.
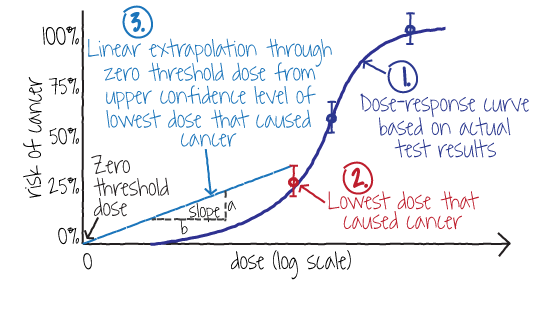
Figure 2. The Linearized Multistage Model is used to extrapolate cancer risk from a dose-response curve using the cancer slope factor
(Image Source: NLM)
(Image Source: NLM)
EPA uses the Linearized Multistage Model (LMS) illustrated in Figure 2 to conduct its cancer risk assessments. It yields a cancer slope factor, known as the q1* (pronounced "Q1-star"), which can be used to predict cancer risk at a specific dose. It assumes linear extrapolation with a zero dose threshold from the upper confidence level of the lowest dose that produced cancer in an animal test or in a human epidemiology study.
Other Models
Other models that have been used for cancer assessments include:
Application of Models to Estimate Chemical Concentrations in Drinking Water
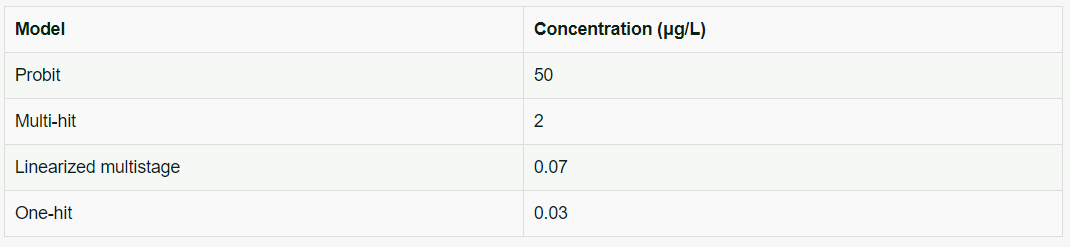
The chemical chlordane has been found to cause a lifetime risk of one cancer death in a million persons. Different cancer risk assessment models vary in their estimates of drinking water concentrations for chlordane as illustrated in Table 2:
Other Models
Other models that have been used for cancer assessments include:
- One-hit model, which assumes there is a single stage for cancer and that one molecular event induces a cell transformation. This is a very conservative model.
- Multi-hit model, which assumes several interactions are needed before a cell can be transformed. This is one of the least conservative models.
- Probit model, which assumes log normal distribution (Probit) for tolerances of exposed population. This model is sometimes used, but generally considered inappropriate for assessing cancer risk.
- Physiologically Based Pharmacokinetic (PBPK) Models, which incorporate pharmacokinetic and mechanistic data into the extrapolation process. This model requires extensive data and is becoming commonly used.
Application of Models to Estimate Chemical Concentrations in Drinking Water
The chemical chlordane has been found to cause a lifetime risk of one cancer death in a million persons. Different cancer risk assessment models vary in their estimates of drinking water concentrations for chlordane as illustrated in Table 2:
Table 2. Estimates of drinking water chlordane concentrations by various cancer assessment models
PBPK models are relatively new and are being employed when biological data are available. They quantitate the absorption of a foreign substance, its distribution, metabolism, tissue compartments, and elimination. Some compartments store the chemical (such as bone and adipose tissue) whereas others biotransform or eliminate it (such as liver or kidney). All these biological parameters are used to derive the target dose and comparable human doses.
Noncarcinogenic Risk Assessment
Historically, the Acceptable Daily Intake (ADI) procedure has been used to calculate permissible chronic exposure levels for humans based on noncarcinogenic effects. The ADI is the amount of a chemical to which a person can be exposed each day for a long time (usually lifetime) without suffering harmful effects. It is determined by applying safety factors (to account for the uncertainty in the data) to the highest dose in human or animal studies that has been demonstrated not to cause toxicity (NOAEL).
The EPA has slightly modified the ADI approach and calculates a Reference Dose (RfD) as the acceptable safety level for chronic noncarcinogenic and developmental effects. Similarly, the ATSDR calculates Minimal Risk Levels (MRLs) for noncancer endpoints.
The critical toxic effect used in the calculation of an ADI, RfD, or MRL is the serious adverse effect that occurs at the lowest exposure level. It may range from lethality to minor toxic effects. It is assumed that humans are as sensitive as the animal species unless evidence indicates otherwise.
Assessment of Chronic Exposures
In determining the ADIs, RfDs or MRLs, the NOAEL is divided by safety factors (uncertainty factors) in order to provide a margin of safety for allowable human exposure.
Noncarcinogenic Risk Assessment
Historically, the Acceptable Daily Intake (ADI) procedure has been used to calculate permissible chronic exposure levels for humans based on noncarcinogenic effects. The ADI is the amount of a chemical to which a person can be exposed each day for a long time (usually lifetime) without suffering harmful effects. It is determined by applying safety factors (to account for the uncertainty in the data) to the highest dose in human or animal studies that has been demonstrated not to cause toxicity (NOAEL).
The EPA has slightly modified the ADI approach and calculates a Reference Dose (RfD) as the acceptable safety level for chronic noncarcinogenic and developmental effects. Similarly, the ATSDR calculates Minimal Risk Levels (MRLs) for noncancer endpoints.
The critical toxic effect used in the calculation of an ADI, RfD, or MRL is the serious adverse effect that occurs at the lowest exposure level. It may range from lethality to minor toxic effects. It is assumed that humans are as sensitive as the animal species unless evidence indicates otherwise.
Assessment of Chronic Exposures
In determining the ADIs, RfDs or MRLs, the NOAEL is divided by safety factors (uncertainty factors) in order to provide a margin of safety for allowable human exposure.
When a NOAEL is not available, a LOAEL can be used to calculate the RfD.
An additional safety factor is included if a LOAEL is used. A Modifying Factor of 0.1–10 allows risk assessors to use scientific judgment in upgrading or downgrading the total uncertainty factor based on the reliability and quality of the data. For example, if a particularly good study is the basis for the risk assessment, a modifying factor of <1 may be used. If a poor study is used, a factor of >1 can be incorporated to compensate for the uncertainty associated with the quality of the study.
An additional safety factor is included if a LOAEL is used. A Modifying Factor of 0.1–10 allows risk assessors to use scientific judgment in upgrading or downgrading the total uncertainty factor based on the reliability and quality of the data. For example, if a particularly good study is the basis for the risk assessment, a modifying factor of <1 may be used. If a poor study is used, a factor of >1 can be incorporated to compensate for the uncertainty associated with the quality of the study.
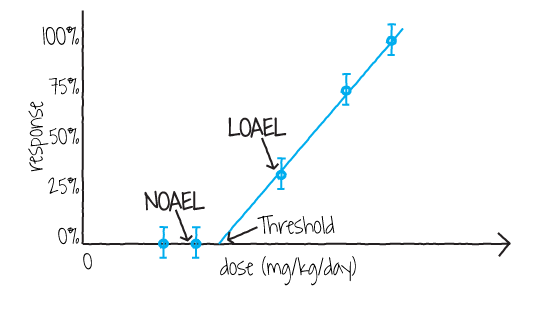
Figure 3. Dose-response curve for noncarcinogenic effects
(Image Source: NLM)
(Image Source: NLM)
Figure 3 above shows a dose-response curve for noncarcinogenic effects which also identifies the NOAEL and LOAEL. Any toxic effect might be used for the NOAEL/LOAEL so long as it is the most sensitive toxic effect and considered likely to occur in humans.
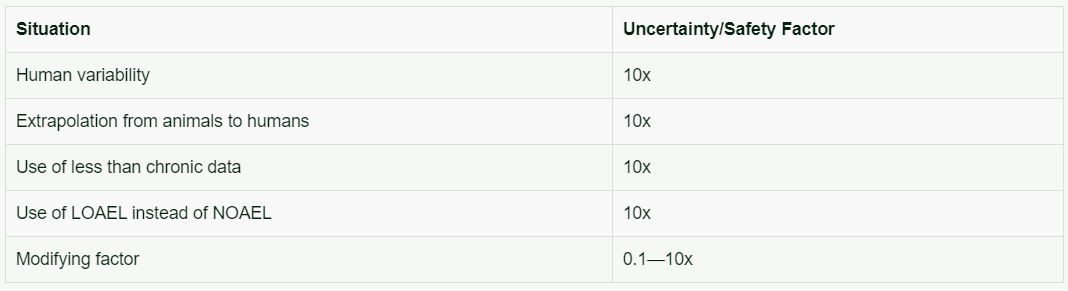
The Uncertainty Factors or Safety Factors used to derive an ADI or RfD are listed in Table 3.
The Uncertainty Factors or Safety Factors used to derive an ADI or RfD are listed in Table 3.
Table 3. Uncertainty/Safety factors used to derive an Acceptable Daily Intake (ADI) or Reference Dose (RfD)
The modifying factor is used only in deriving EPA Reference Doses. The number of factors included in calculating the ADI or RfD depends upon the study used to provide the appropriate NOAEL or LOAEL.
The general formula for deriving the RfD is:
The general formula for deriving the RfD is:
The more uncertain or unreliable the data become, the higher the total uncertainty factor that is applied. An example of an RfD calculation is provided below. A subchronic animal study with a LOAEL of 50 mg/kg/day was used in the numerator. Uncertainty factors used in the denominator are 10 for human variability, 10 for an animal study, 10 for less than chronic exposure, and 10 for use of an LOAEL instead of a NOAEL.
In addition to chronic effects, RfDs can also be derived for other long-term toxic effects, including developmental toxicity.
Traditionally, the NOAEL method has been used to determine the point of departure (POD - represents a dose derived from observed data that is associated with an extra risk for a specific endpoint) from animal toxicology data for use in risk assessments. However, this approach has limitations such as a strict dependence on the dose selection, dose spacing, and sample size of the study from which the critical effect has been identified. Also, using the NOAEL does not take into consideration the shape of the dose-response curve and other related information.
Traditionally, the NOAEL method has been used to determine the point of departure (POD - represents a dose derived from observed data that is associated with an extra risk for a specific endpoint) from animal toxicology data for use in risk assessments. However, this approach has limitations such as a strict dependence on the dose selection, dose spacing, and sample size of the study from which the critical effect has been identified. Also, using the NOAEL does not take into consideration the shape of the dose-response curve and other related information.
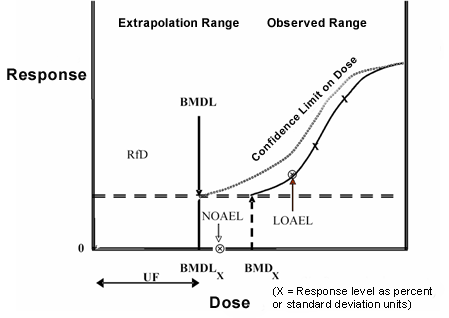
Benchmark Dose Method
The benchmark dose (BMD) method, first proposed as an alternative in the 1980s, addresses many limitations of the NOAEL method. It is less dependent on dose selection and spacing and takes into account the shape of the dose-response curve (Figure 4). In addition, the estimation of a BMD 95% lower bound confidence limit (BMDL) results in a POD that appropriately accounts for study quality (i.e., sample size). With the availability of user-friendly BMD software programs, including the EPA’s Benchmark Dose Software (BMDS), the BMD has become the method of choice for many health organizations worldwide.
Figure 4. Extrapolated values using the benchmark dose method reflect the shape of a dose-response curve
(Image Source: EPA)
(Image Source: EPA)
Assessment of Noncancer Toxicity Effects
While the Agency for Toxic Substances and Disease Registry (ATSDR) does not conduct cancer risk assessments, it does derive Minimal Risk Levels (MRLs) for noncancer toxicity effects (such as birth defects or liver damage). The MRL is defined as an estimate of daily human exposure to a substance that is likely to be without an appreciable risk of adverse effects over a specified duration of exposure. For inhalation or oral routes, MRLs are derived for acute (14 days or less), intermediate (15–364 days), and chronic (365 days or more) durations of exposures.
The method used to derive MRLs is a modification of the EPA's RfD methodology. The primary modification is that the uncertainty factors of 10 may be lower, either 1 or 3, based on scientific judgment. These uncertainty factors are applied for human variability, interspecies variability (extrapolation from animals to humans), and use of a LOAEL instead of NOAEL. As in the case of RfDs, the product of uncertainty factors multiplied together is divided into the NOAEL or LOAEL to derive the MRL.
Assessment of Acute or Short-Term Exposures
Risk assessments are also conducted to derive permissible exposure levels for acute or short-term exposures to chemicals. Health Advisories (HAs) are determined for chemicals in drinking water. HAs are the allowable human exposures for 1 day, 10 days, longer-term, and lifetime durations. The method used to calculate HAs is similar to that for the RfDs using uncertainty factors. Data from toxicity studies with durations of length appropriate to the HA are being developed.
Assessment of Occupational Exposures
For occupational exposures, Permissible Exposure Levels (PELs), Threshold Limit Values (TLVs), and National Institute for Occupational Safety and Health (NIOSH) Recommended Exposure Levels (RELs) are developed. They represent dose levels that will not produce adverse health effects from repeated daily exposures in the workplace. The method used to derive is conceptually the same. Safety factors are used to derive the PELs, TLVs, and RELs.
Conversion of Animal Doses to Human Dose Equivalents
Animal doses must be converted to human dose equivalents. The human dose equivalent is based on the assumption that different species are equally sensitive to the effects of a substance per unit of body weight or body surface area.
Historically, the FDA used a ratio of body weights of humans to animals to calculate the human dose equivalent. The EPA has used a ratio of surface areas of humans to animals to calculate the human dose equivalent. Some current approaches include multiplying the animal dose by the ratio of human to animal body weight raised to either the 2/3rd or 3/4th power (to convert from body weight to surface area). Toxicologists and risk assessors should check to make sure that the approach they are using is the one mandated or recommended by the regulatory agency of most relevance to their efforts.
Allowable Exposures to Contamination Sources
The last step in risk assessment is to express the risk in terms of allowable exposure to a contaminated source. Risk is expressed in terms of the concentration of the substance in the environment where human contact occurs. For example, the unit for assessing risk in air is risk per mg/m3 whereas the unit for assessing risk in drinking water is risk per mg/L.
For carcinogens, the media risk estimates are calculated by dividing cancer slope factors by 70 kg (average weight of a man) and multiplying by 20 m3/day (average inhalation rate of an adult) or 2 liters/day (average water consumption rate of an adult).
While the Agency for Toxic Substances and Disease Registry (ATSDR) does not conduct cancer risk assessments, it does derive Minimal Risk Levels (MRLs) for noncancer toxicity effects (such as birth defects or liver damage). The MRL is defined as an estimate of daily human exposure to a substance that is likely to be without an appreciable risk of adverse effects over a specified duration of exposure. For inhalation or oral routes, MRLs are derived for acute (14 days or less), intermediate (15–364 days), and chronic (365 days or more) durations of exposures.
The method used to derive MRLs is a modification of the EPA's RfD methodology. The primary modification is that the uncertainty factors of 10 may be lower, either 1 or 3, based on scientific judgment. These uncertainty factors are applied for human variability, interspecies variability (extrapolation from animals to humans), and use of a LOAEL instead of NOAEL. As in the case of RfDs, the product of uncertainty factors multiplied together is divided into the NOAEL or LOAEL to derive the MRL.
Assessment of Acute or Short-Term Exposures
Risk assessments are also conducted to derive permissible exposure levels for acute or short-term exposures to chemicals. Health Advisories (HAs) are determined for chemicals in drinking water. HAs are the allowable human exposures for 1 day, 10 days, longer-term, and lifetime durations. The method used to calculate HAs is similar to that for the RfDs using uncertainty factors. Data from toxicity studies with durations of length appropriate to the HA are being developed.
Assessment of Occupational Exposures
For occupational exposures, Permissible Exposure Levels (PELs), Threshold Limit Values (TLVs), and National Institute for Occupational Safety and Health (NIOSH) Recommended Exposure Levels (RELs) are developed. They represent dose levels that will not produce adverse health effects from repeated daily exposures in the workplace. The method used to derive is conceptually the same. Safety factors are used to derive the PELs, TLVs, and RELs.
Conversion of Animal Doses to Human Dose Equivalents
Animal doses must be converted to human dose equivalents. The human dose equivalent is based on the assumption that different species are equally sensitive to the effects of a substance per unit of body weight or body surface area.
Historically, the FDA used a ratio of body weights of humans to animals to calculate the human dose equivalent. The EPA has used a ratio of surface areas of humans to animals to calculate the human dose equivalent. Some current approaches include multiplying the animal dose by the ratio of human to animal body weight raised to either the 2/3rd or 3/4th power (to convert from body weight to surface area). Toxicologists and risk assessors should check to make sure that the approach they are using is the one mandated or recommended by the regulatory agency of most relevance to their efforts.
Allowable Exposures to Contamination Sources
The last step in risk assessment is to express the risk in terms of allowable exposure to a contaminated source. Risk is expressed in terms of the concentration of the substance in the environment where human contact occurs. For example, the unit for assessing risk in air is risk per mg/m3 whereas the unit for assessing risk in drinking water is risk per mg/L.
For carcinogens, the media risk estimates are calculated by dividing cancer slope factors by 70 kg (average weight of a man) and multiplying by 20 m3/day (average inhalation rate of an adult) or 2 liters/day (average water consumption rate of an adult).
Knowledge Check (Solutions on next page)
1) The default procedures used to extrapolate from high to low doses primarily depend upon the:
a) Potency of the substance
b) Rate of lethality in laboratory animals
c) Genotoxic carcinogenicity of the substance
2) According to EPA, a substance is classified as likely to be carcinogenic to humans when:
a) There is strong evidence of human carcinogenicity
b) Evidence is adequate to demonstrate potential carcinogenicity to humans, but not strongly enough to definitively classify as carcinogenic
c) The weight of evidence suggests human carcinogenicity, but the data are determined not to be sufficient for a stronger conclusion
d) Robust data lead to the conclusion that a substance is clearly carcinogenic to humans
3) The primary cancer risk assessment model used by the EPA is known as the:
a) Linearized Multistage Model (LMS)
b) Probit Model
c) Physiologically Based Pharmacokinetic Model (PB-PK)
4) The Acceptable Daily Intake (ADI) is calculated by:
a) Dividing the NOAEL by safety factors
b) Dividing the NOAEL by the LOAEL
c) Multiplying the RfD by a modifying factor
d) Linear extrapolation from the LOAEL to the zero intercept
5) Animal doses must be converted to human dose equivalents for risk assessment. When doing this, toxicologists and risk assessors must:
a) Multiply the animal dose by the ratio of human to animal body weight raised to the 2⁄3 power
b) Ensure they use the conversion method mandated or recommended by the regulatory agency most relevant to their efforts
c) Multiply the animal dose by the ratio of human to animal body weight raised to the 3⁄4 power
6) Minimal Risk Levels (MRLs) are derived:
a) Similarly to deriving the RfD, but with a potentially lower uncertainty factor
b) By multiplying the cancer slope factor by the lowest exposure dose
c) By multiplying the LOAEL by safety factors